RETeval: An Essential Tool for Glaucoma Care

Dr. Andre Lenoir describes how he went from considering electroretinography as an antiquated technology to regarding it as an essential tool in modern optometry.
Expand Your Opportunities for Profitability

According to Dr. Christopher Lopez, the RETeval handheld ERG device is the electrodiagnostic equipment of choice for the modern day optometrist.
Photopic Negative Response Using RETeval System Provides Objective RNFL Information
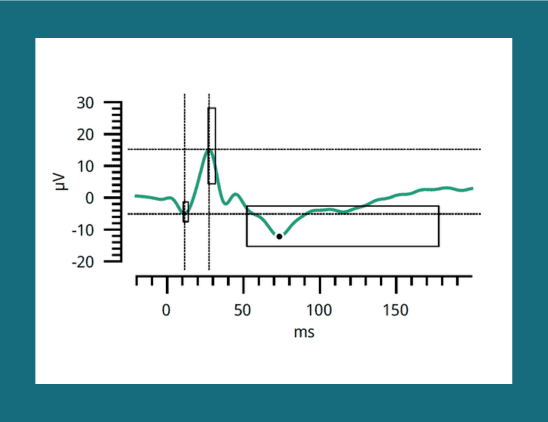
Researchers from Athens investigated structural and functional changes in the optic nerve in patients with early glaucoma under treatment.
From Urban Academia To Rural Private Practice

Drs. Dorothy Hitchmoth and Julie Rodman are using handheld ERG to embrace new guidance from the diabetic retinopathy taskforce with a goal to elevate the standard of care.
LKC Technologies Wins Sustainable Exhibitor Award

LKC was recognized with a Sustainable Exhibitor Award by the European Society of Cataract & Refractive Surgeons (ESCRS) at their 41st Congress, held in Vienna, Austria.
Task Force Releases Consensus Document for Managing DR in Optometry

A panel of optometrists endeavored to develop a set of guidelines that will guide peers in simplifying and standardizing DR assessment and management to avoid preventable vision loss.
Increase Access to Eye Care at Your Practice

Dr. Francis Bynum discusses how to make eye care more accessible, how to select devices based on ROI and patient needs, and how to train staff to use them.
How We Spurred 5% Growth Over Last Year in Diabetes Services
Dr. Melissa Richard discusses how her practice is expanding comprehensive diabetic eyecare services, highlighting key actions, investments, and strategies to enhance patient care.
Simplify Grading and Risk Assessment in Diabetic Retinopathy

In this Review of Optometry feature, leading optometrists propose that DR is graded at the time of diagnosis and at each subsequent visit, and that retinal cell function is quantified.
Diabetic Retinopathy Management Protocols for Optometry

In this July 2023 issue of Review of Optometry, leading optometrists explain lay out fundamental principles for diabetic retinopathy management and share valuable advice.
The Three Biggest Ways ERG Changed My Practice

Read this June 2023 Women in Optometry feature to learn why Dr. Frances Bynum cannot imagine practicing without her RETeval handheld ERG.
Retina Technology To Upgrade Patient and Practice Health
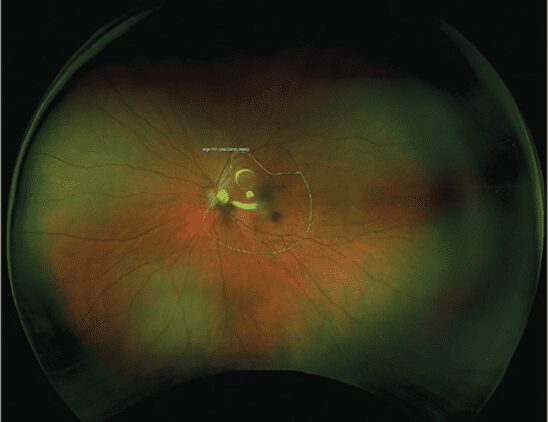
In the June 2023 issue of Optometric Management, Tim Earley, OD reviews posterior segment technology that improves patient experience and …
