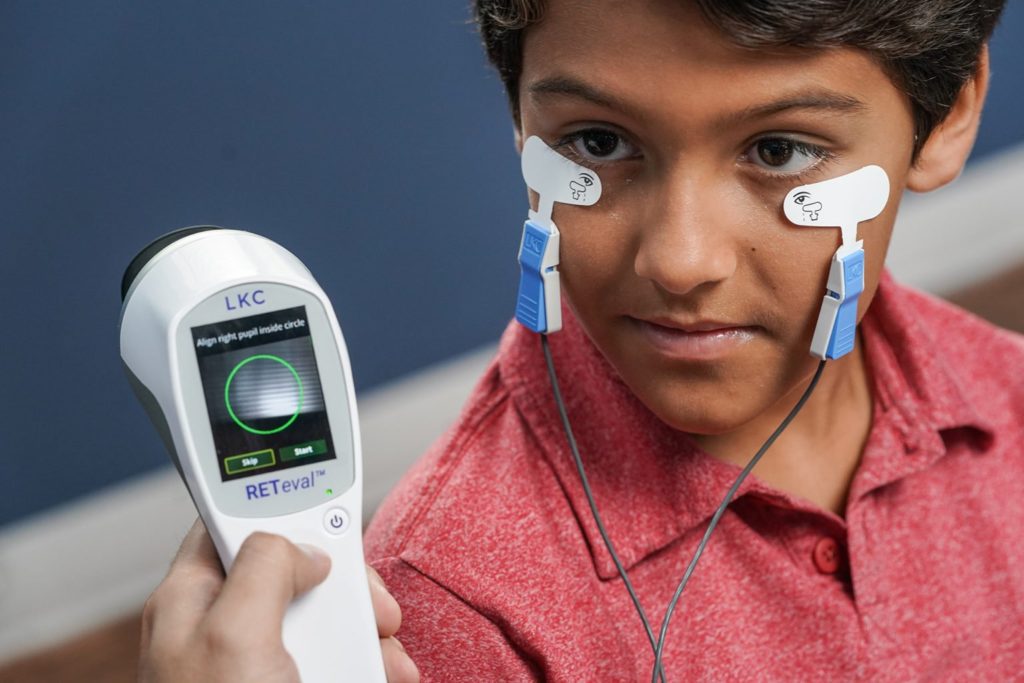
Originally founded as LKC Systems in 1975 by Jerome Leight, Sigmund Krassowsky, and Frank Chen, the company known today as LKC Technologies has spent the past 35+ years demystifying visual electrophysiology, helping clinicians diagnose and manage eye disease.

At LKC, we aim to preserve vision by offering technology that makes functional retinal assessments accessible everywhere, for everyone.
As a global organization, LKC’s committed representatives are driven to make a difference through innovative technology and quality customer service.

![]() Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Substance Tables:
The table below lists substances which may be contained within LKC’s RETeval and RETevet products. Substances listed as Type 1 are within permissible levels in one or more of LKC’s products. Substances listed as Type 2 are used in the production of some components used in LKC products and may be present at trace levels, but are typically destroyed during processing.
RETeval and RETevet Devices
| Substance | CAS # | Type | Listed as causing: |
| Nickel | 7440-02-0 | 1 | Cancer |
| Acrylonitrile | 107-13-1 | 2 | |
| Ethylbenzine | 100-41-4 | 2 | |
| Crystaline Silica | 14808-60-7 | 1 | |
| Lead | 7439-92-1 | 1 | Cancer Developmental Toxicity Male Reproductive Toxicity Female Reproductive Toxicity |
| Methylene Chloride | 75-09-2 | 2 | Cancer Female Reproductive Toxicity |
| Bisphenol A | 80-05-7 | 2 | |
| N-Hexane | 110-54-3 | 2 | Male Reproductive Toxicity |
