
Michael Cymbor, OD, FAAO
Nittany Eye Associates
(State College, PA)
By Michael Cymbor, OD, FAAO, Dorothy L. Hitchmoth, OD, FAAO, and J. James Thimons, OD, FAAO
Originally published in the January 2023 issue of Review of Optometry
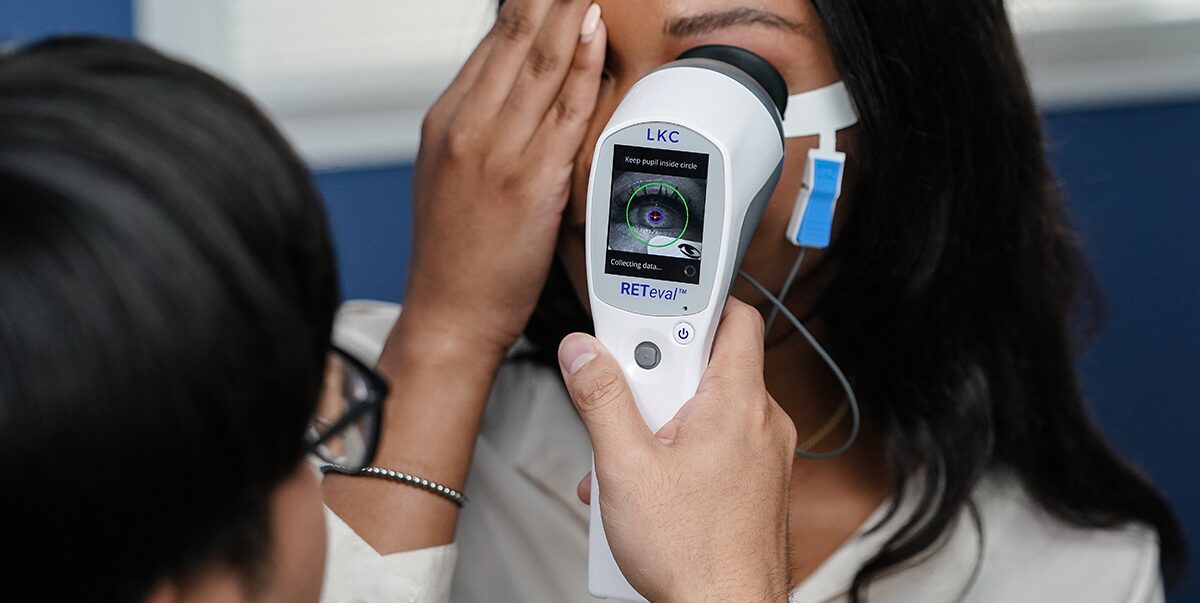
What type of ERG device do you use in your practice?
Dr. Thimons: We use the RETeval® device because it’s powerful and fits in the palm of your hand. In fact, it’s the only FDA-cleared, portable, non-mydriatic electroretinography (ERG) testing instrument on the market in the US. It is a major departure from traditional electroretinography and represents a technology that has evolved from large, expensive, and complicated to portable, affordable and easy-to-use and interpret.
How does ERG help you manage patients with diabetes?
Dr. Hitchmoth: Early detection of retinal abnormalities is a critical step in preventing vision loss. That’s why the RETeval has been a game changer in how I care for my patients who have diabetes. In an assessment of RETeval’s ability to evaluate diabetic retinopathy, the advantages included earlier detection of retinal dysfunction, lower investment costs, and less required reading knowledge than traditionally-used imaging techniques.1 The way we care for patients is advancing beyond fundus imaging.
Dr. Cymbor: The DR Assessment Protocol provides a superior risk assessment for progression. A score of 23.5 or higher indicates an 11-fold risk of requiring intervention within 3 years.2 As diabetic patients worsen into moderate and severe nonproliferative disease, it may become challenging to determine the best time to refer to a retinal specialist. The DR Assessment Protocol helps me to clarify the correct time to refer, enhancing patient outcomes.
Is ERG needed if you have access to a good structural imaging device?
Dr. Thimons: Combining structural and functional information provides better results. Electroretinography objectively evaluates the functional abnormalities of the retina, while structural imaging shows the anatomy of the retinal tissue. While both functional and structural assessments have their benefits, functional changes generally appear well before structural changes. In studies comparing ERG and structural imaging’s abilities to evaluate sight-threatening diabetic retinopathy, RETeval ERGs outperformed the traditional imaging techniques in predicting which patients would later need medical intervention.2,3

How does ERG help you manage glaucoma patients?
Dr. Cymbor: With the variability and length of subjective visual field testing, I appreciate having a quick and objective way to measure visual function. The RETeval PhNR test objectively measures the ganglion cells’ function by evaluating the electrical activity of the cells to a light stimulus. Knowing about the function of the ganglion cells assists in the detection and tracking of glaucomatous changes. The test also has high repeatability, independent of media opacities.4-6 PhNR testing with the RETeval device provides sensitve, objective tracking of retinal changes for more informed follow-up, even where no changes in visual fi eld or RNFL could be detected.7
How time-consuming is ERG testing?
Dr. Hitchmoth: Your technician can perform the test in 2-3 minutes, making it one of the most efficient tools available in our practice. Plus, it’s completely objective and doesn’t cause patient frustration. Patients tolerate the test and love the fact that they do not have to respond or provide the “right answer”.
How do you bill for ERG?
Dr. Cymbor: There are more than 670 codes you can select from to bill for ERG. In our practice, we most commonly use CPT code 92273, billing the RETeval as a reimbursable test for DR. Some optometrists use the DR protocol to screen all patients with diabetes, whether or not they’ve been diagnosed with retinopathy, but this application is not reimbursable using this code.

Nittany Eye Associates
(State College, PA)

Hitchmoth Eye Care
(New London, NH)

Ophthalmic Consultants of Connecticut
(Fairfield, CT)
![]() Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Substance Tables:
The table below lists substances which may be contained within LKC’s RETeval and RETevet products. Substances listed as Type 1 are within permissible levels in one or more of LKC’s products. Substances listed as Type 2 are used in the production of some components used in LKC products and may be present at trace levels, but are typically destroyed during processing.
RETeval and RETevet Devices
| Substance | CAS # | Type | Listed as causing: |
| Nickel | 7440-02-0 | 1 | Cancer |
| Acrylonitrile | 107-13-1 | 2 | |
| Ethylbenzine | 100-41-4 | 2 | |
| Crystaline Silica | 14808-60-7 | 1 | |
| Lead | 7439-92-1 | 1 | Cancer Developmental Toxicity Male Reproductive Toxicity Female Reproductive Toxicity |
| Methylene Chloride | 75-09-2 | 2 | Cancer Female Reproductive Toxicity |
| Bisphenol A | 80-05-7 | 2 | |
| N-Hexane | 110-54-3 | 2 | Male Reproductive Toxicity |
