Animal ERG Testing
Made Easy
Full-field ERG testing for animal and veterinary ophthalmology that’s fully portable and easy to use.
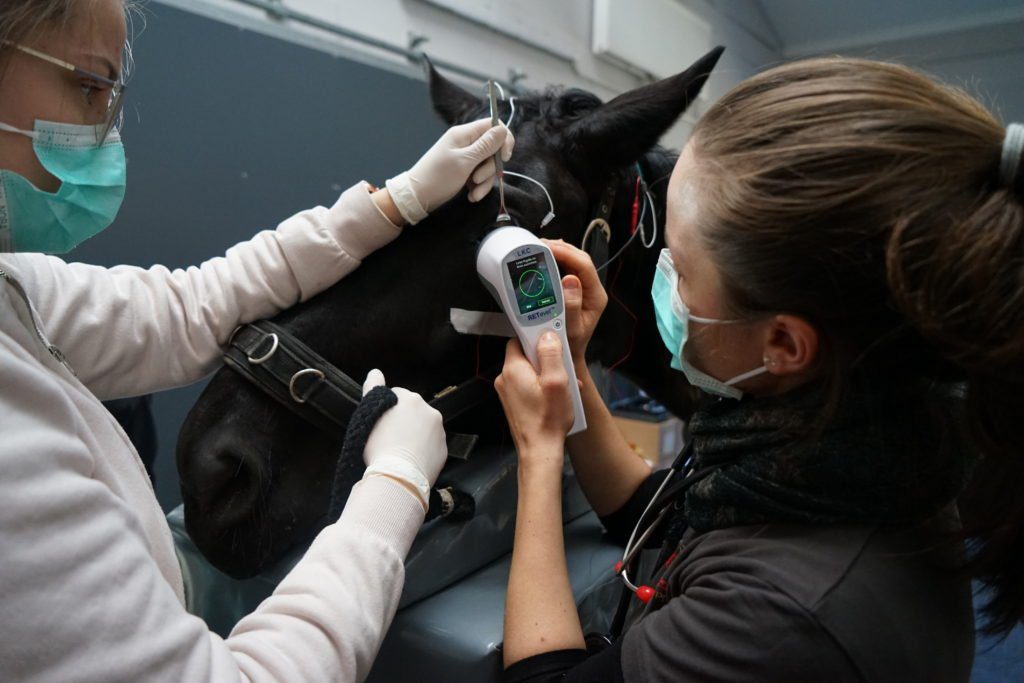
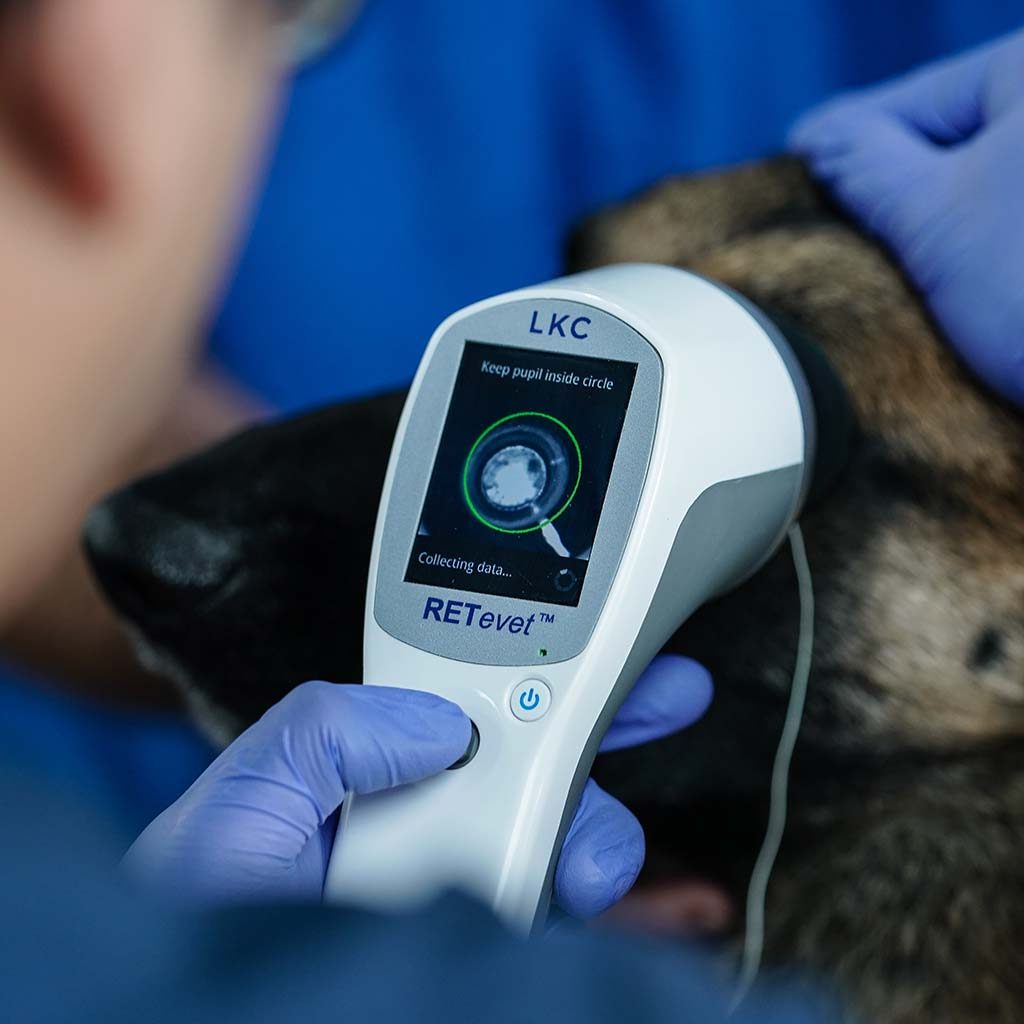
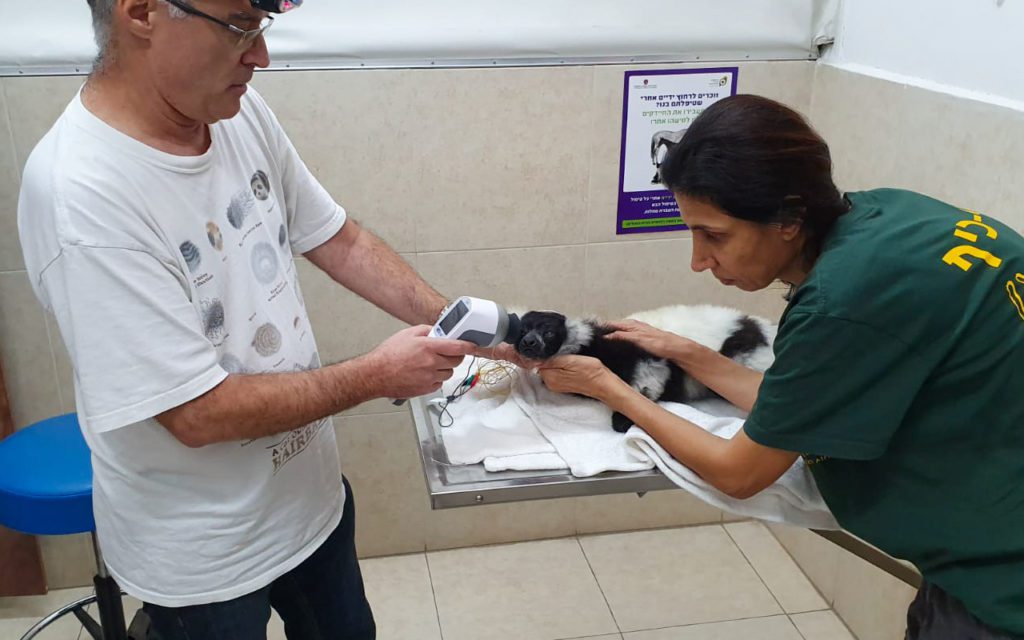


Courtesy of Dr. Göritz from the Leibniz-Institut für Zoo- und Wildtierforschung


Detectar o estresse funcional
Antecede o dano estrutural.
ERG is used to assess retinal function in animals and is an extremely valuable diagnostic tool for veterinary ophthalmologists and researchers. ERG testing is indispensable before cataract surgery to rule out retinal degenerations and the RETevet is an invaluable tool for diagnosing various inherited and acquired retinal diseases — especially when ophthalmoscopic abnormalities are not present.
ERG testing can be conducted with the RETevet despite the presence of media opacities, and it provides veterinarians with vital information about retinal function.

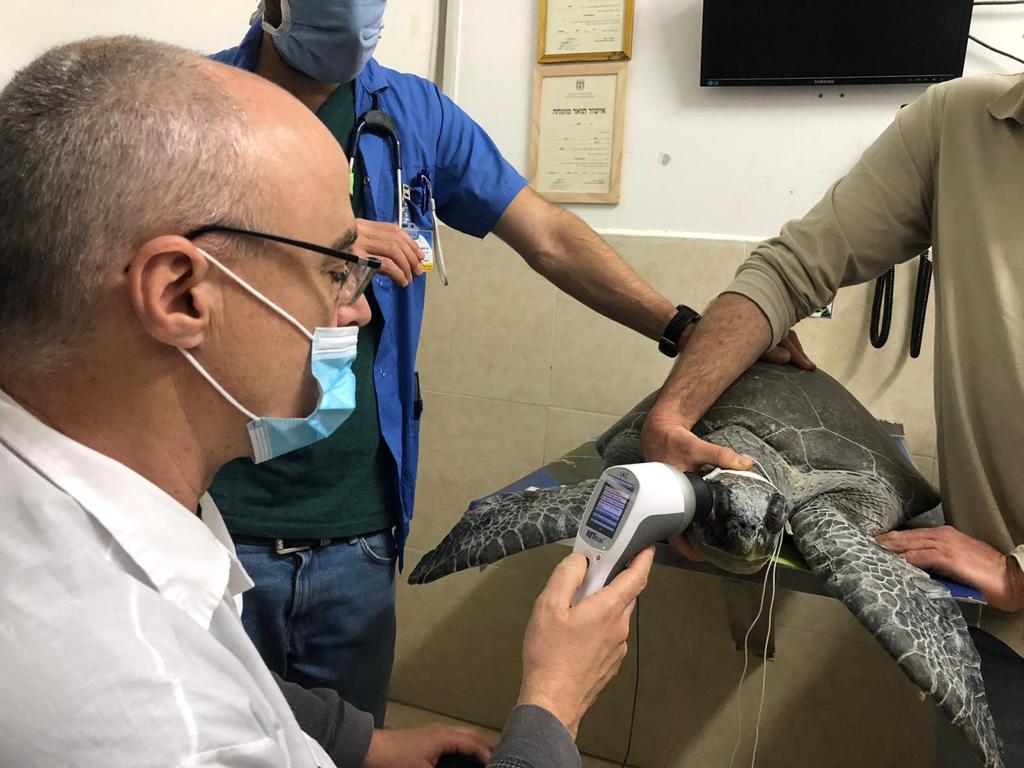
Detect various inherited and acquired retinal diseases
Predict the outcome of cataract surgery and disease progression
Differentiate between retinal and post-retinal causes of animal blindness
Ensure the absence of retinal disease for breeding, research, or certification purposes
Animal ERG testing with the RETevet can quickly and easily detect and determine a wide range of diagnostic information, aiding veterinarians and researchers with comprehensive data on how the retina is functioning and/or changing.
RETevet testing uses ECVO and ISCEV non-human protocols

Early identification and monitoring of inherited retinal conditions such as Progressive Retinal Atrophy (PRA), hemeralopia (day blindness), and Congenital Stationary Night Blindness (CSNB), and more.

Assist in differential diagnoses of Sudden Acquired Retinal Degeneration (SARDS) or Optic Neuritis. Evaluate retinal detachments, and conditions such as Feline central retinal degeneration (FCRD).

Quickly evaluate retinal conditions with the only test (ERG) not affected by media opacities. Diagnose retinal disorders even when no ophthalmoscopic abnormalities are detected.
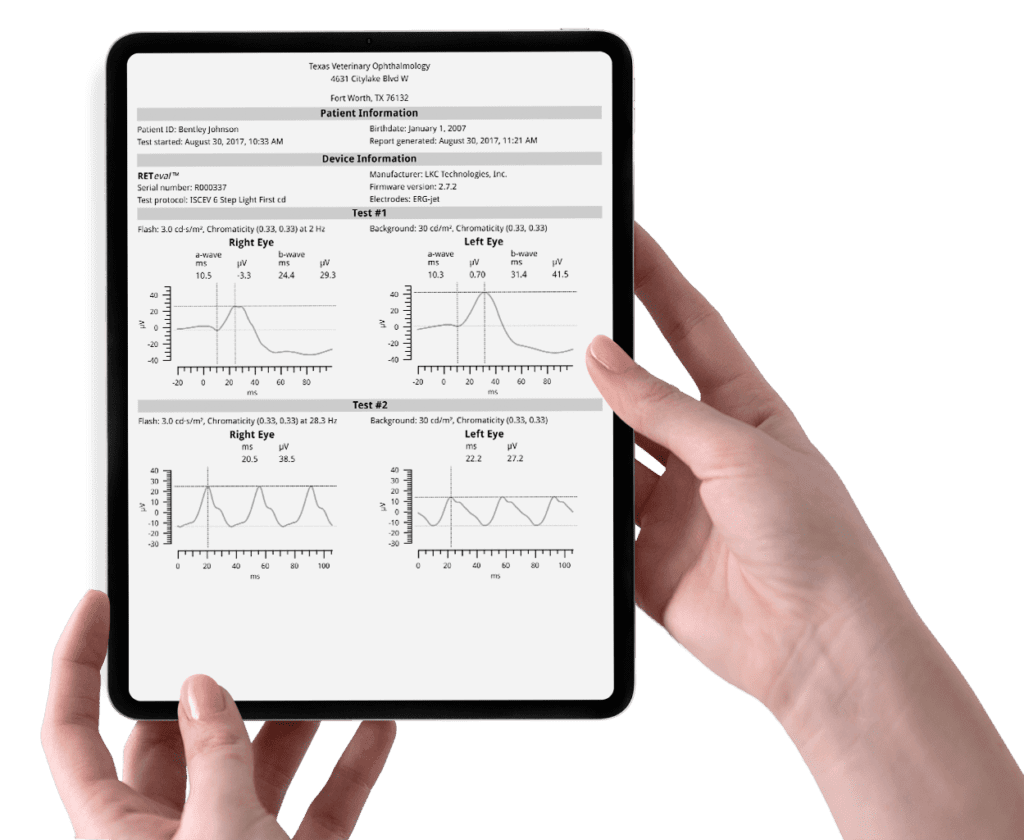
Clear reports with numeric values available in 17 languages help to confirm or exclude retina disease.
Its clear content is a valuable tool to communicate with the pet owner about the prognosis of disease, surgical outcomes, cataract recomendations, and breeding certification.





A nonhuman primate model of inherited retinal disease

Chromatic electroretinography in non-human primates using a novel algorithm
Obter mais informações ou solicitar uma demonstração ou orçamento do dispositivo RETevet device
![]() Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Substance Tables:
The table below lists substances which may be contained within LKC’s RETeval and RETevet products. Substances listed as Type 1 are within permissible levels in one or more of LKC’s products. Substances listed as Type 2 are used in the production of some components used in LKC products and may be present at trace levels, but are typically destroyed during processing.
RETeval and RETevet Devices
| Substance | CAS # | Type | Listed as causing: |
| Nickel | 7440-02-0 | 1 | Cancer |
| Acrylonitrile | 107-13-1 | 2 | |
| Ethylbenzine | 100-41-4 | 2 | |
| Crystaline Silica | 14808-60-7 | 1 | |
| Lead | 7439-92-1 | 1 | Cancer Developmental Toxicity Male Reproductive Toxicity Female Reproductive Toxicity |
| Methylene Chloride | 75-09-2 | 2 | Cancer Female Reproductive Toxicity |
| Bisphenol A | 80-05-7 | 2 | |
| N-Hexane | 110-54-3 | 2 | Male Reproductive Toxicity |
