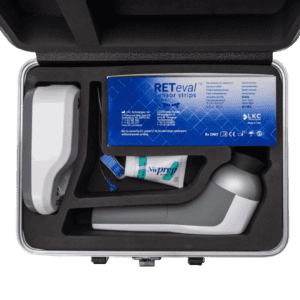
The Carrying Case Will Hold:
- RETeval or RETevet device
- Device docking station (for charging)
- AC adapter (for charging)
- Electrode connecting cable, power and USB cable
- One box of 50 Sensor Strip electrodes
The case provides a convenient way to carry the RETeval® or RETevetTM device between testing locations. Everything needed for up to 50 tests is held in a small, durable case with dedicated cushioned compartments.
The custom foam cushioning of the carrying case is constructed of special non-shedding foam to help keep the device optics and ganzfeld bowl dust and lint free.

| Handling | Single comfortable handle |
| Security | Locking clasp with key |
| Width | 12.4″ (315 mm) |
| Depth | 9.0″ (230 mm) |
| Height | 5.5″ (140 mm) |
| Weight (empty) | 2.3 lb (1,05 kg) |
| Weight (full) | 4.3 lb (1,95 kg) |
![]() Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Substance Tables:
The table below lists substances which may be contained within LKC’s RETeval and RETevet products. Substances listed as Type 1 are within permissible levels in one or more of LKC’s products. Substances listed as Type 2 are used in the production of some components used in LKC products and may be present at trace levels, but are typically destroyed during processing.
RETeval and RETevet Devices
| Substance | CAS # | Type | Listed as causing: |
| Nickel | 7440-02-0 | 1 | Cancer |
| Acrylonitrile | 107-13-1 | 2 | |
| Ethylbenzine | 100-41-4 | 2 | |
| Crystaline Silica | 14808-60-7 | 1 | |
| Lead | 7439-92-1 | 1 | Cancer Developmental Toxicity Male Reproductive Toxicity Female Reproductive Toxicity |
| Methylene Chloride | 75-09-2 | 2 | Cancer Female Reproductive Toxicity |
| Bisphenol A | 80-05-7 | 2 | |
| N-Hexane | 110-54-3 | 2 | Male Reproductive Toxicity |
