
Mario Zanolli es un oftalmólogo pediátrico de Clinica Alemana de Santiago y Hospital Roberto del Río, Profesor Adjunto de la Universidad del Desarrollo. Hizo su formación en la Fundación Oftalmológica Los Andes y luego un fellow en Genética Ocular en el Hospital Wills de Philadelphia, donde fue co-autor del Manual Wills de Oculogenética.
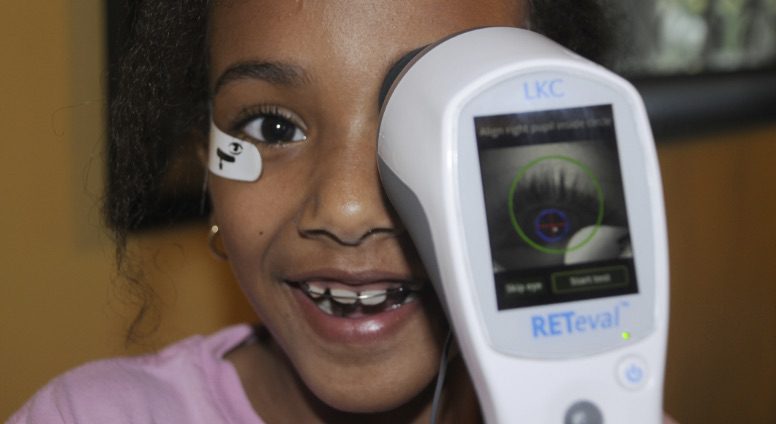
La electrofisiología es en muchos casos una prueba obligada dentro de la oftalmología pediátrica, proporcionando un conocimiento profundo sobre la función de la retina. El dispositivo RETeval® ERG es un dispositivo ERG portátil, que utiliza los protocolos ISCEV, fácil de usar y con especial beneficio en el segmento pediátrico.
En este webinar se hará una breve introducción a la electrofisiología, seguida de la presentación de varios casos pediátrico, demostrando cómo utilizar e interpretar los resultados electrorretinográficos. Tras la presentación habrá tiempo para preguntas y respuestas sobre electrofisiología.
Electrophysiology provides a vast amount of information about retina function. It’s therefore a required test for many applications within pediatric ophthalmology.
The RETeval® ERG device is an ISCEV compliant, easy to use, hand-held ERG device that is especially useful in a pediatric clinical setting.
Join Dr. Mario Zanolli as he presents a brief introduction into electrophysiology, followed by a presentation of several case presentations. The goal? Demonstrating how to use and interpret the results quickly and easily.
After the presentation, there will be time for questions and answers from the audience.
![]() Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Substance Tables:
The table below lists substances which may be contained within LKC’s RETeval and RETevet products. Substances listed as Type 1 are within permissible levels in one or more of LKC’s products. Substances listed as Type 2 are used in the production of some components used in LKC products and may be present at trace levels, but are typically destroyed during processing.
RETeval and RETevet Devices
| Substance | CAS # | Type | Listed as causing: |
| Nickel | 7440-02-0 | 1 | Cancer |
| Acrylonitrile | 107-13-1 | 2 | |
| Ethylbenzine | 100-41-4 | 2 | |
| Crystaline Silica | 14808-60-7 | 1 | |
| Lead | 7439-92-1 | 1 | Cancer Developmental Toxicity Male Reproductive Toxicity Female Reproductive Toxicity |
| Methylene Chloride | 75-09-2 | 2 | Cancer Female Reproductive Toxicity |
| Bisphenol A | 80-05-7 | 2 | |
| N-Hexane | 110-54-3 | 2 | Male Reproductive Toxicity |
