With more than 200,000 US cases per year, cataracts are the leading cause of blindness in the world and according to YourSightMatters.com, 24 million Americans over the age of 40 are affected by them.
Examining retina function can be a daunting task in the presence of cataracts, but it is important to move forward with an electroretinogram (ERG) to test the retina and determine whether it is still functional. With a dysfunctional retina, there is no reason for patients to undergo cataract surgery, which in the long run will help save time and money for physicians and their patients. If other abnormalities in the retina are found during the ERG testing phase, other ocular diseases may be determined, such as diabetic retinopathy or retinal vein occlusions which will result in different treatments.
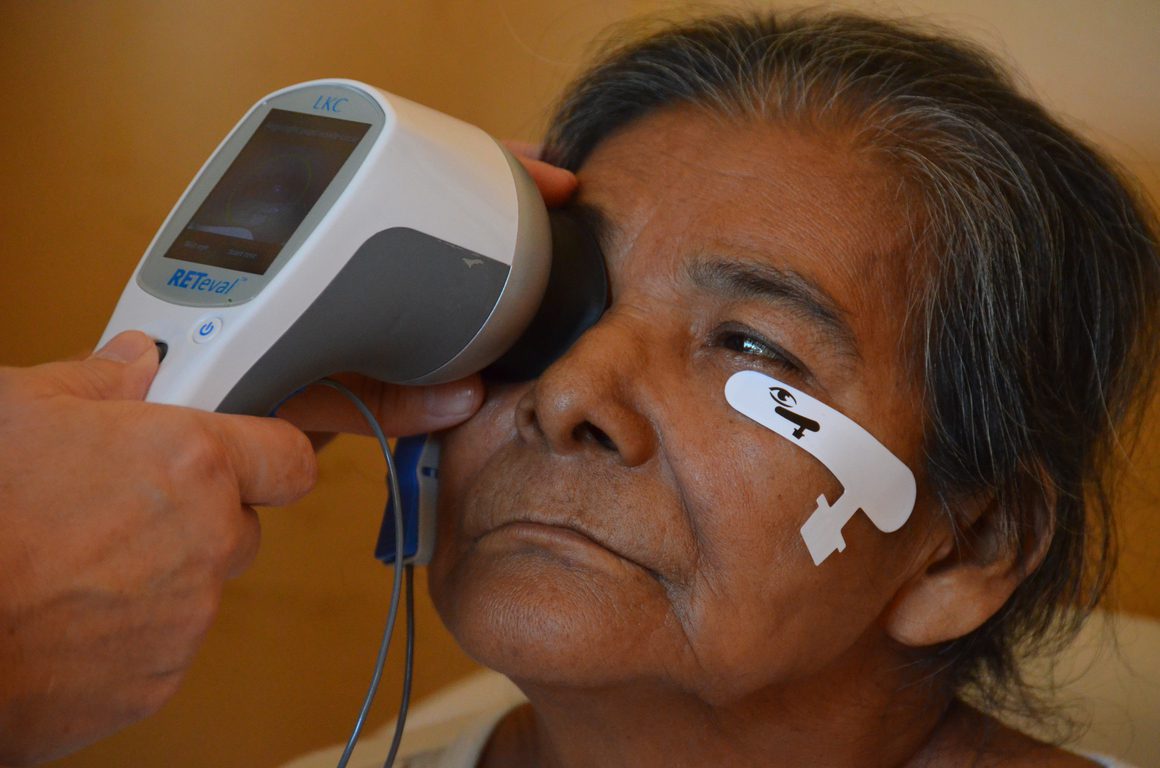
The RETeval® device is a practical method to assess the retinal function in eyes with cataracts because it can be performed rapidly, about 1 min/eye, without mydriasis and topical anesthetics.
In a study done in 2015, Effects of cataracts on flicker electroretinograms recorded with RETeval system: new mydriasis-free ERG device, the RETeval device examined 82 eyes of 60 patients with 43 having Grade 2* cataracts and 17 having Grade 3* cataracts. During this study, 52 eyes were also examined of 38 patients who underwent cataract surgery previously, to see the comparison of all three groups during the flicker ERG.
This study indicates that the presence of Grade 2 or more cataracts affects both the amplitude and the implicit time of the flicker ERGs therefore affecting recordings from the RETeval device. The presence of cataracts should be taken into consideration when interpreting flicker ERG recordings.
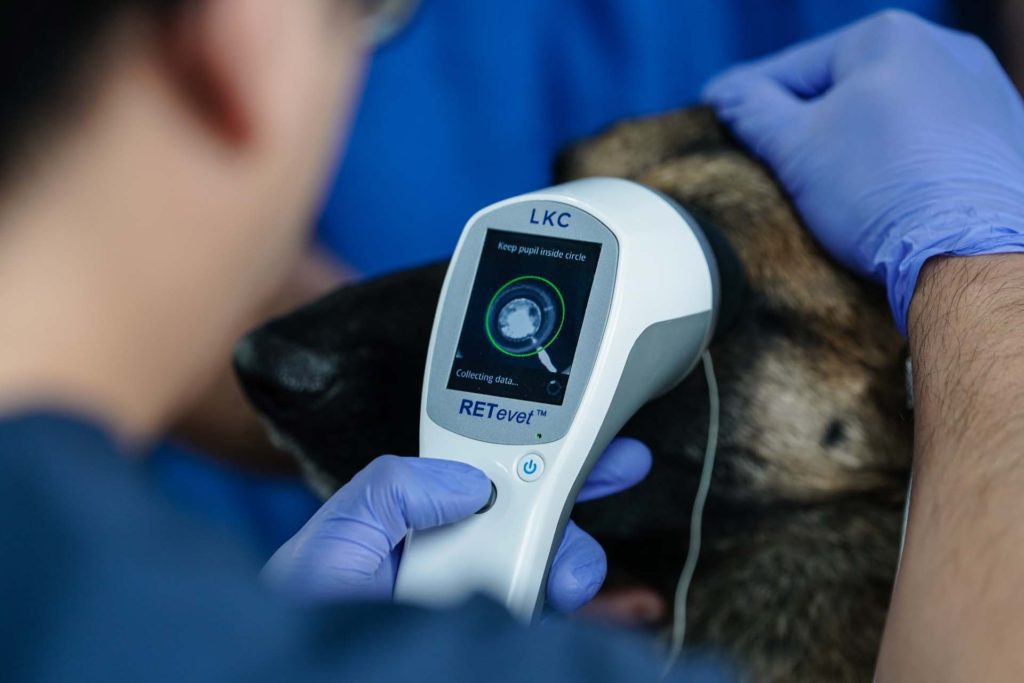
There are approximately 75 million dogs in the United States and 45% of all households in the country have at least one dog as a pet. This causes the demand for veterinary health providers to increase drastically. With over 100,000 veterinarians in 60,000 clinics across the country, these health professionals are looking for safe and effective technologies to aid in the diagnosis of disease.
Cataracts affect 50% of dogs older than 9.4 years regardless of their breed. Certain cases such as the American Cocker Spaniel and Miniature Poodle can develop genetically triggered cataracts.
The treatment for these family pets is the same of human treatment, which is undergoing an intraocular lens implantation. To optimize the success in vision post-surgery, it is strongly recommended to take a full assessment of the retina. Basically, a near-normal ERG test means that removal of the cataract during surgery will improve vision, if the ERG test shows a substantially decreasing retina function, surgery may not improve vision due to other ocular diseases.
Overall, an electroretinogram (ERG) is an essential pre-surgical evaluation tool to efficiently discriminate between suitable and unsuitable cataract surgery candidates. The RETeval® and RETevet™ devices are easily adaptable in any practice to measure retina function to establish a diagnosis and further treatment of the patient.
* Based on the Emery-Little classification: Surgical techniques, complications and results. Phacoemulsification and Aspiration of Cataract.
![]() Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Substance Tables:
The table below lists substances which may be contained within LKC’s RETeval and RETevet products. Substances listed as Type 1 are within permissible levels in one or more of LKC’s products. Substances listed as Type 2 are used in the production of some components used in LKC products and may be present at trace levels, but are typically destroyed during processing.
RETeval and RETevet Devices
| Substance | CAS # | Type | Listed as causing: |
| Nickel | 7440-02-0 | 1 | Cancer |
| Acrylonitrile | 107-13-1 | 2 | |
| Ethylbenzine | 100-41-4 | 2 | |
| Crystaline Silica | 14808-60-7 | 1 | |
| Lead | 7439-92-1 | 1 | Cancer Developmental Toxicity Male Reproductive Toxicity Female Reproductive Toxicity |
| Methylene Chloride | 75-09-2 | 2 | Cancer Female Reproductive Toxicity |
| Bisphenol A | 80-05-7 | 2 | |
| N-Hexane | 110-54-3 | 2 | Male Reproductive Toxicity |
