Approximately 1.4 million people live in nursing homes across the US, with approximately 57% of these residents suffering from visual impairment 1,2. Approximately 40% of these visual impairments are classified as “avoidable blindness” when receiving appropriate care 3. However, gaining access to eye care is a challenge for residents and their families 4.
This is why we developed a program with 360 Care that offers optometric services to nursing homes across 18 states. Providing optometric care directly within a nursing home can create several challenges for an Optometrist. One of these challenges is the availability of portable diagnostic instruments.
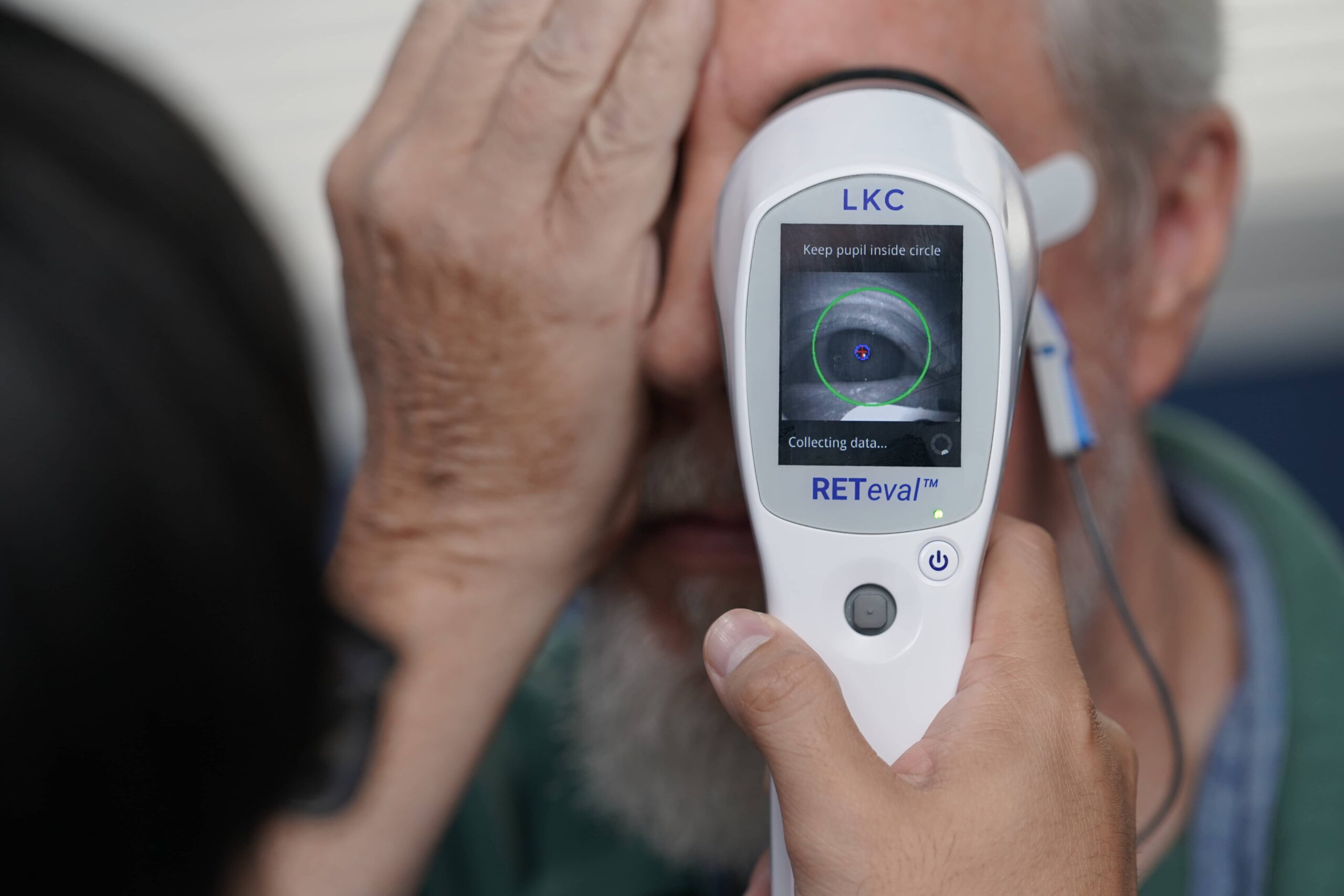
Recently, a portable, hand-held device, with no direct contact required on the eye itself, has become available to measure the function of retinal cells objectively. I believe that this new device has a great deal of potential in a nursing home setting.
An electroretinogram (ERG) measures the electrical response of the photoreceptor cells, inner retinal cells, and ganglion cells after a light stimulus. Deviations in the time (implicit time) and strength (amplitude) of the response help to diagnose and monitor several diseases commonly present in nursing home populations.
Although ERG has demonstrated usefulness in assisting in diagnosing and monitoring diseases such as diabetic retinopathy and glaucoma, previous device size and usability limitations have restricted the spread of ERG into the clinic. It is important to note that ERG is an objective measurement of retinal function. It is not dependent on the cognitive abilities of the patient and requires only minimal cooperation. As opposed to retinal imaging, ERG measurements are not sensitive to cataracts and allow us a comprehensive understanding of retinal function in the largest group of our patients 3.
With the advent of a small, easy to use and truly portable device such as RETeval® device, ERG can become a valuable diagnostic tool for patients in nursing homes.
This is why we developed a program with 360 Care that offers optometric services to nursing homes across 18 states. Providing optometric care directly within a nursing home can create several challenges for an Optometrist. One of these challenges is the availability of portable diagnostic instruments.
Recently, a portable, hand-held device, with no direct contact required on the eye itself, has become available to measure the function of retinal cells objectively. I believe that this new device has a great deal of potential in a nursing home setting.
The RETeval device comes with a test protocol called the DR Assessment. The DR Assessment test with the DR Score has been clinically validated in multiple cross-sectional and longitudinal studies to aid in the detection of Vision Threatening Diabetic Retinopathy (VTDR) and predict the risk of DR disease progression. A recent study showed that patients having a DR score equal to or greater than 23.5 were 11 times more likely to have a future ocular intervention than patients having scores less than 23.5. 7
Now, that I have a RETeval device with me during nursing home visits, I can quickly and comfortably monitor any resident with known or suspected diabetic retinopathy. I apply the skin electrodes, select the appropriate test from the device menu, follow the instructions on the device, and get results immediately after the test.
The easy-to-read report is color-coded, helping me quickly see abnormal values compared to the included reference data. In case of a high score, I can test further, see the patient more frequently, or refer them for treatment.
This sample report of an 85-year-old patient with a DR score of 24.6 reveals that this patient is at a greater risk of developing VTDR.
As with diabetic retinopathy, the first measurement and analysis of glaucoma with ERG took place in the late 1940s 8. More recent evidence suggests that a specific component of the flash ffERG electrical response, the Photopic Negative Response (PhNR), is valid for assisting in assessing glaucomatous eyes 9,10. A recent study reported sensitivity as high as 85.4% and specificity as high as ≥ 95% 10.
This high diagnostic accuracy gives me a higher confidence level when I cannot measure visual field defects but still need to screen for glaucoma. The PhNR test is as easy to perform as the test for diabetic retinopathy.
The PhNR implicit times and amplitudes for both eyes are abnormal when compared against the age-adjusted reference data. These values are highlighted in red and indicate retinal ganglion cell stress, damage, and possible disease progression.
"Having the RETeval system for my nursing home visits has been a great addition to my practice. It has allowed me to easily assess the visual function of my patients where it would typically be difficult, if not impossible."
Dr. Andrew Feltz, OD

Dr. Andrew Feltz graduated from The Ohio State College of Optometry in 1995 and completed his residency training at the Cleveland VA Medical Center in Ocular Disease and Hospital Based Optometry. After a couple of years of general practice in Columbus, Ohio, he co-founded a mobile healthcare company – OnSight Healthcare. By 2016, the company had expanded to 6 states and serviced over 700 nursing homes. Andy has a passion for volleyball and has been a youth volleyball coach for over 10 years.
![]() Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Substance Tables:
The table below lists substances which may be contained within LKC’s RETeval and RETevet products. Substances listed as Type 1 are within permissible levels in one or more of LKC’s products. Substances listed as Type 2 are used in the production of some components used in LKC products and may be present at trace levels, but are typically destroyed during processing.
RETeval and RETevet Devices
| Substance | CAS # | Type | Listed as causing: |
| Nickel | 7440-02-0 | 1 | Cancer |
| Acrylonitrile | 107-13-1 | 2 | |
| Ethylbenzine | 100-41-4 | 2 | |
| Crystaline Silica | 14808-60-7 | 1 | |
| Lead | 7439-92-1 | 1 | Cancer Developmental Toxicity Male Reproductive Toxicity Female Reproductive Toxicity |
| Methylene Chloride | 75-09-2 | 2 | Cancer Female Reproductive Toxicity |
| Bisphenol A | 80-05-7 | 2 | |
| N-Hexane | 110-54-3 | 2 | Male Reproductive Toxicity |
