We prepare each offer individually depending on your needs, and you can expect to receive it in your inbox within five business days. Thank you for your patience.
If you’d like to learn more about our product systems, you can explore here:
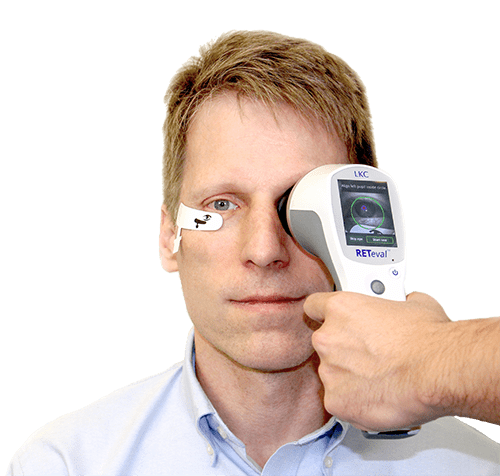
The RETeval® flicker ERG assessments provide more than ten flicker assessment protocols, including one specifically designed for vision-threatening diabetic retinopathy (DR). This specific DR protocol objectively measures risk progression. The RETeval uses patented adhesive Sensor Strip skin electrodes which eliminate the need for corneal contact, increasing patient comfort.
Read more
The RETeval Complete is a fully ISCEV-compliant, full-field flash and flicker system optimized for easy assessment of retinal function in acquired ischaemic, optic nerve, and hereditary diseases. The handheld device uses patented adhesive Sensor Strip skin electrodes which eliminate the need for corneal contact and can be especially beneficial when testing children.
Read more
The UTAS system provides high-quality multifocal and pattern stimuli for assessing macular and optic nerve function in a straightforward, ISCEV-compliant method. This technology is optimal for assessments that require a more detailed ERG/VEP analysis, such as hydroxychloroquine toxicity or optic neuritis. The UTAS system also provides an optimal tool set for researchers examining the macula and optic nerve.
Read more
With the combination of the UTAS system and the RETeval Complete device, you’ll have the full range of ERG and VEP testing required for a majority of clinical applications that occur in a routine clinic. All Ganzfeld ERG/VEP assessments can be completed easily with the entirely handheld RETeval Complete, and all multifocal and pattern ERG/VEP tests for optic nerve diseases and toxicities are included in the UTAS system. Both technologies utilize the Sensor Strip adhesive skin electrodes for optimized patient comfort and convenient test set-up.
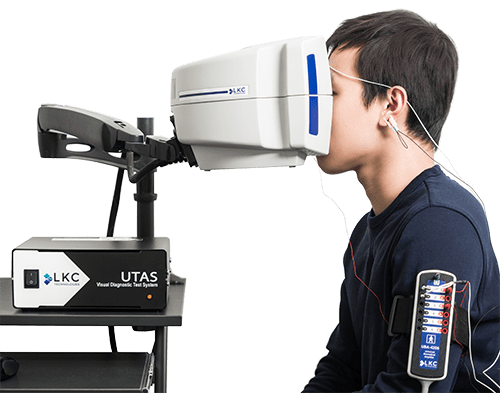
The UTAS SunBurstTM includes all Ganzfeld ERG and VEP assessments as well as EOG testing for detailed electrophysiologic examinations in a clinic or research setting.
Read more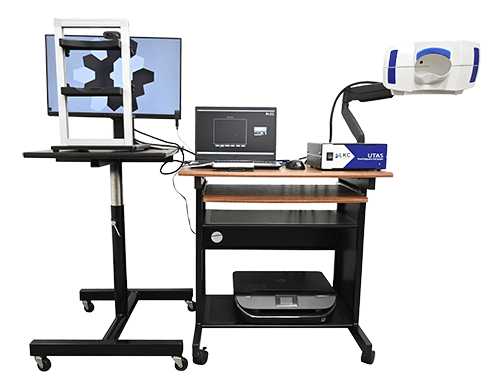
With the UTAS SunBurst and multifocal ERG, the full range of electrophysiology testing is covered in one system, providing high quality and flexibility for each test. The UTAS can also be used with patented Sensor Strip adhesive skin electrodes if desired.
Read more
The full range of visual electrophysiology is available with the UTAS SunBurst, multifocal and pattern ERG, dark adaptation, and EOG modules in a single platform to maximize research capabilities and help clinicians diagnose the most complex diseases.
Read more![]() Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Substance Tables:
The table below lists substances which may be contained within LKC’s RETeval and RETevet products. Substances listed as Type 1 are within permissible levels in one or more of LKC’s products. Substances listed as Type 2 are used in the production of some components used in LKC products and may be present at trace levels, but are typically destroyed during processing.
RETeval and RETevet Devices
| Substance | CAS # | Type | Listed as causing: |
| Nickel | 7440-02-0 | 1 | Cancer |
| Acrylonitrile | 107-13-1 | 2 | |
| Ethylbenzine | 100-41-4 | 2 | |
| Crystaline Silica | 14808-60-7 | 1 | |
| Lead | 7439-92-1 | 1 | Cancer Developmental Toxicity Male Reproductive Toxicity Female Reproductive Toxicity |
| Methylene Chloride | 75-09-2 | 2 | Cancer Female Reproductive Toxicity |
| Bisphenol A | 80-05-7 | 2 | |
| N-Hexane | 110-54-3 | 2 | Male Reproductive Toxicity |
