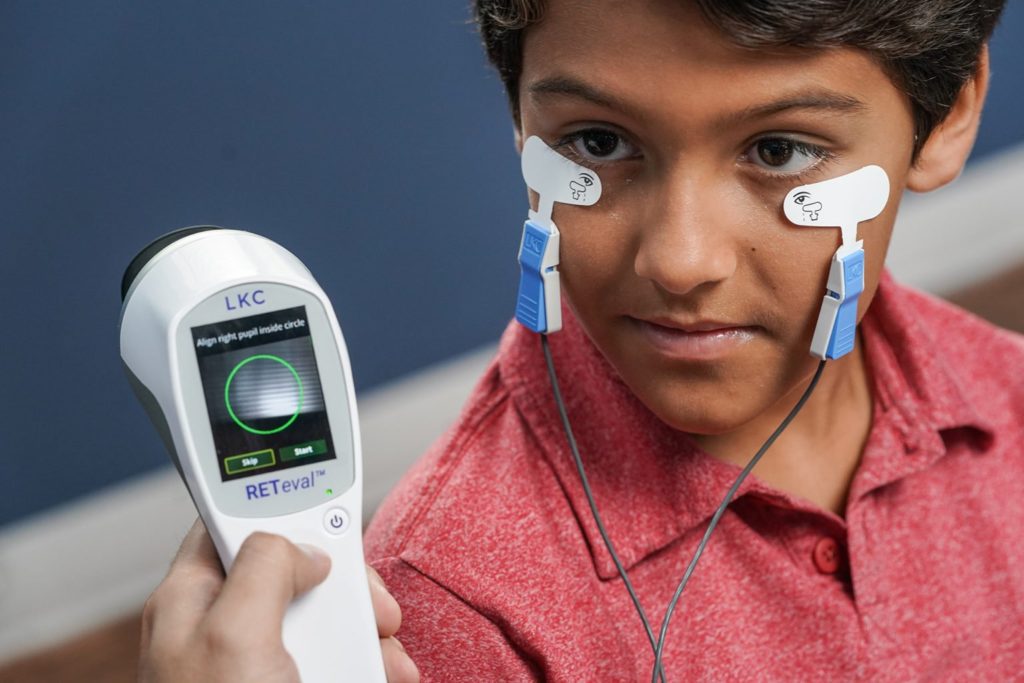
ERG SIMPLIFIED
Electroretinography Coding and Reimbursement Guide
To learn more about other common ICD-10 codes that may be used for additional common protocols associated with the RETeval handheld ERG, please see this guide provided by The Pinnacle Health Group.