Evaluation of light- and dark-adapted ERGs using a mydriasis-free, portable system: clinical classifications and normative data

The purpose of this study was to determine the intra-visit reliability and diagnostic capability of a handheld, mydriasisfree ERG, RETeval…
LKC Staff’s Publication within Top-10 Most Downloaded Papers in 2018

Dr. Quentin Davis and Dr. Olga Krasweska’s peer-reviewed publication -Constant luminance (cd·s/m2) versus constant retinal illuminance (Td·s) stimulation in flicker…
LKC Technologies’ Portable RETeval® Electrodiagnostic Device Adds Arbitrary Waveform Functionality
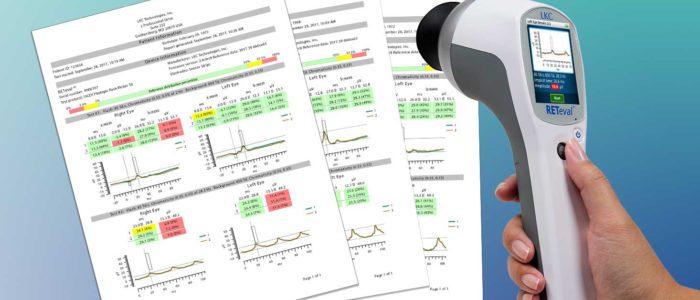
New Protocol Offers More Accurate Testing of the Inner Retina for Research and Clinical Use GAITHERSBURG, MD, May 22, 20…
Role of a Mydriasis-Free, Full-Field Flicker ERG Device in the Detection of Diabetic Retinopathy
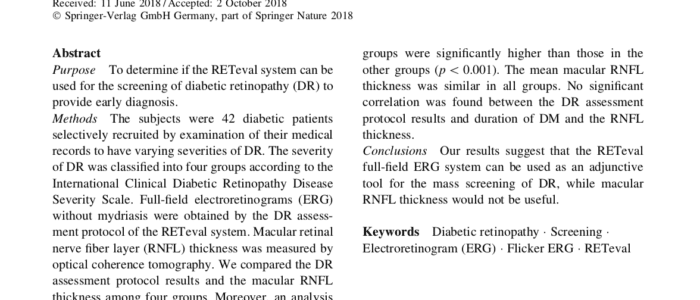
The purpose of this research is to determine if the RETeval system can be used for the screening of diabetic retinopathy (DR)…
LKC Technologies’ Portable RETeval™ Electrodiagnostic Device and Sensor Strips Earn Two United States Patents
Comprehensive electrophysiology system transforms eye care, enabling ophthalmologists and optometrists to conduct tests to assess…
2018 Degirmenci. Role of a mydriasis-free, full-field flicker ERG device…

The purpose of this study is to accelerate the development of new therapies, an inherited retinal degeneration model in a…
Easy and Effective Functional Test for Pediatric Ophthalmology Assessment with RETeval Complete™ Touted by Early Adopters

GAITHERSBURG, Md., March 28, 2016 – Jim Datovech, President of LKC Technologies, Inc. said, “We are learning from a number of…
RETeval™ Device Broadens Ophthalmologists’ Access to Functional Retinal Testing
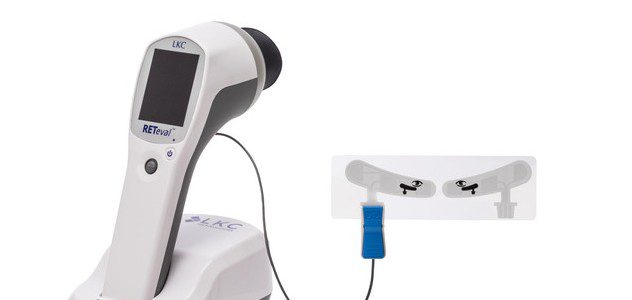
LAS VEGAS, Nov. 13, 2015 — A low cost, handheld visual electrodiagnostic device cleared by the FDA last spring is now available…
RETeval™ Expands Presence at ARVO to Include Testing for Acquired and Inherited Diseases of the Retina
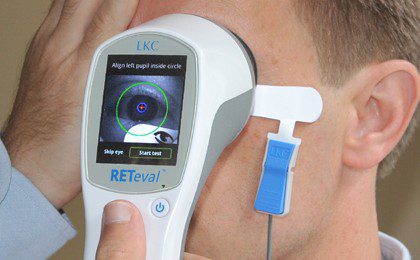
GAITHERSBURG, Md., April 22, 2015 – RETeval – DR™, a Welch Allyn device manufactured by LKC Technologies, Inc., is exclusively available outside…
Welch Allyn and LKC Technologies announce an exclusive distribution agreement for RETeval DR visual electrodiagnostic system

Exclusive Distribution Agreement Announced Welch Allyn and LKC Technologies announce an exclusive distribution agreement for RETeval DR visual electrodiagnostic system…
Promise of Faster, More Accessible Schizophrenia Diagnosis
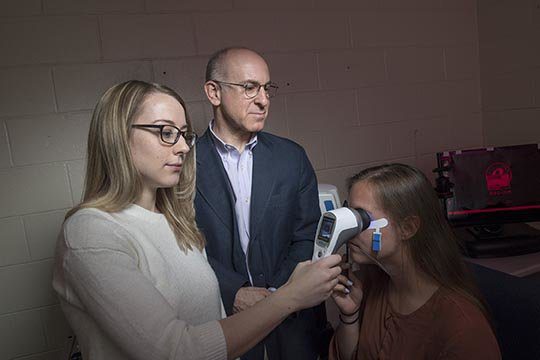
A recent study shows promise of faster, more accessible schizophrenia diagnosis with the hand-held, portable, RETeval device.
LKC Technologies’ Clinical Trial Validates Cost-Effective Alternative to Gold Standard in Diabetic Retinopathy Screening

GAITHERSBURG, Maryland, Sept. 4, 2014 /PRNewswire/ — The EURETINA/ESCRS joint congress scheduled for 12-16 September is the setting for April Maa, MD, Primary…
