Article
Optometric Management
May 2024
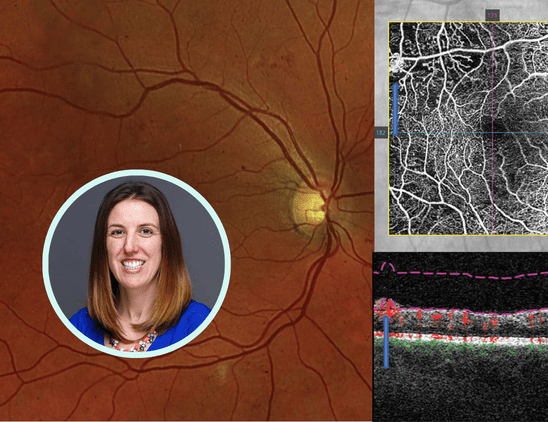
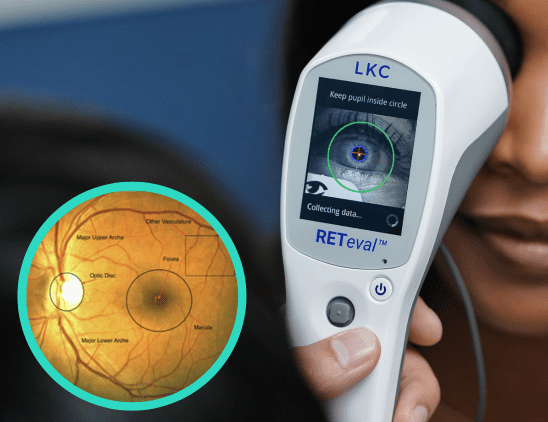
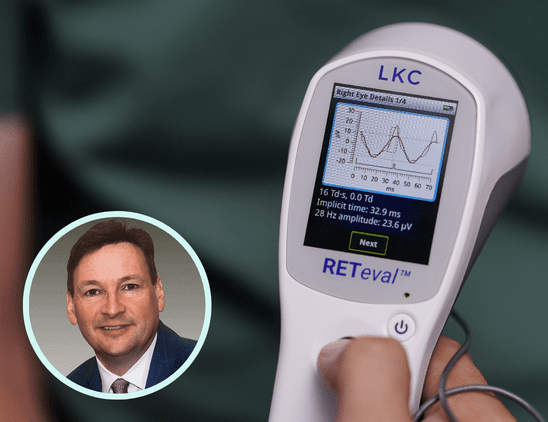
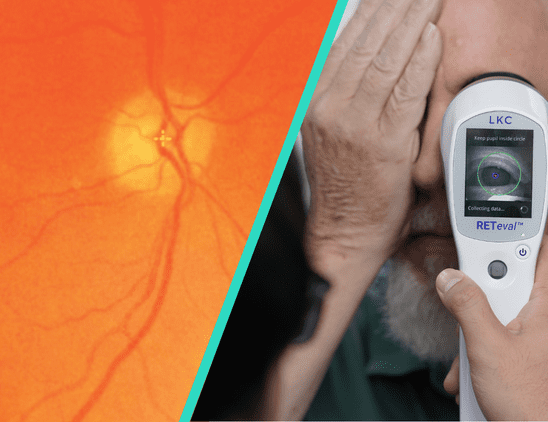
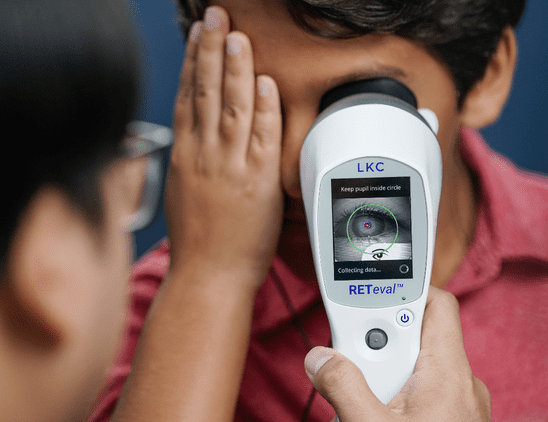
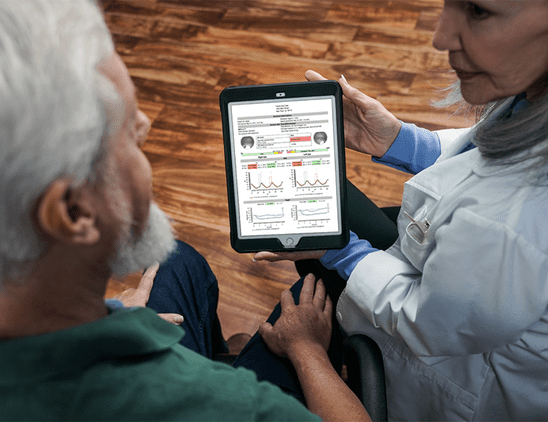
With prevalence of diabetes on the rise, recognizing the early signs of diabetic retinopathy is critical for intervention. In this article, Dr. Anna Bedwell discusses devices that can aid in detection and management of the disease.