Designed with you in Mind
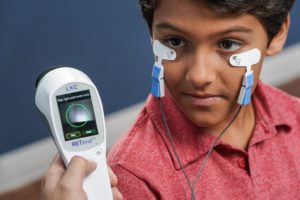
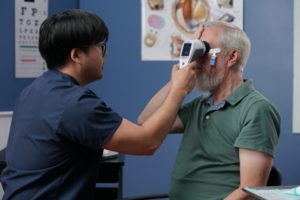
- Testing on both eyes can be completed in only a few minutes
- Portable, handheld device can be used anywhere in your clinic
- Color-coded report simplifies interpretation
- Age-matched reference database helps stratify patient results

Comfortable for Patients
- Patented Sensor Strip™ adhesive skin electrodes save time & reduce hassle
- Flexible Sensor Strip material is gentle on skin with no corneal contact required
- Appropriate for any age without sedation
- Dilation not required

Novel practice builder
- Reimbursable with CPT code 92273
- More than 560 applicable ICD-10 codes
- Seamlessly integrates into clinic workflow with minimal training
- Accessible investment to fuel practice growths