The RETeval® offers a portable, handheld solution that is comfortable for patients, easy for clinicians, and provides an efficient, objective functional assessment of retinal health.
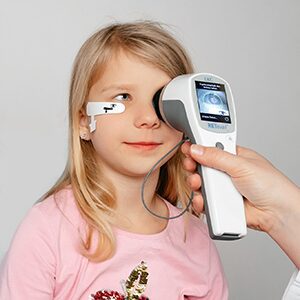
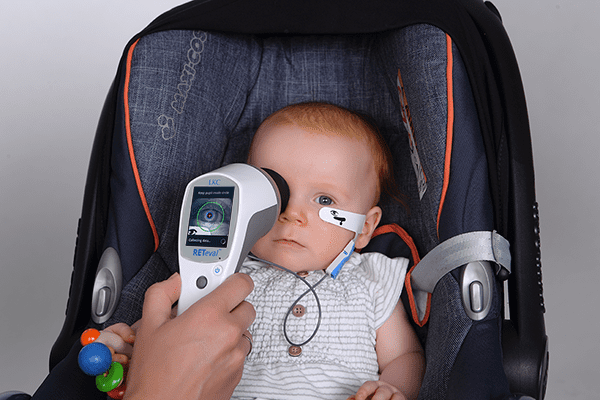
We understand the need for comfortable, efficient testing in a pediatric setting. The handheld RETeval device, together with patented adhesive Sensor Strip skin electrodes, offers an optimized, easy-to-use solution for ERG and VEP testing with flexibility and comfort for patients and clinicians alike.
Adhesive Sensor Strip skin electrodes are available in two sizes for a comfortable fit. These flexible adhesive electrodes move with the patient and are simple to place, allowing consistent, sensitive functional assessment of retinal health.
Sensor Strips are available for purchase in our online store.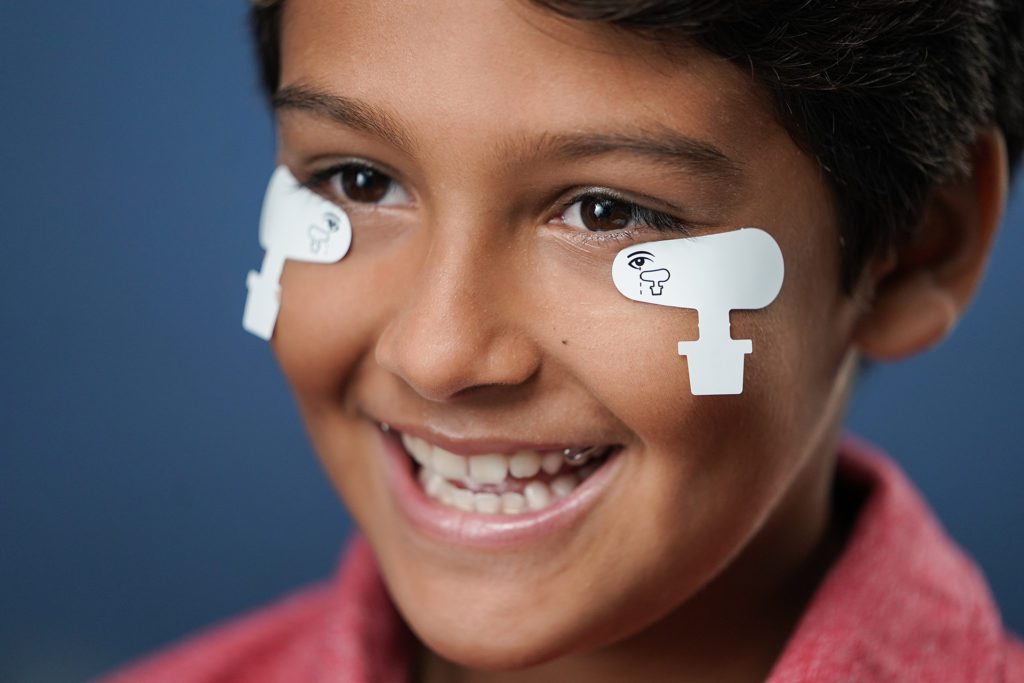
Discover how the RETeval ERG/VEP device, plays a crucial role in understanding the cause of highmyopia in a pediatric patient – all
without sedation.
The Sensor Strip Electrode quality was validated by 14 studies with more than 600 patients. Read a few citations from these studies:

ERG testing on children is difficult as you know. Using a device that is well tolerated and easy on the patient ensures that you get the clinical information you need. See what a few investigators experienced with traditional systems and their electrodes.

“The ophthalmoscopic appearance of his fundus suggested that this boy was also affected with retinitis pigmentosa, but he did not agree to have the ERG examination with the conventional system with contact lens electrodes. The flicker responses in the RETeval showed reduced amplitudes in both eyes (1.1 µV, right eye and 0.56 µV, left eye), which were lower than the mean −2.0 SD of the control eyes.”[33]
“One limitation of the present study is that younger children (4-6 years) were not included in the analysis. This was because most children within this age range could not tolerate the Espion E3 visual electrophysiology system and the DTL electrodes. Subsequently, ERG data for younger children were collected using a hand-held ERG system with skin electrodes (the RETeval visual electrophysiology system, LKC).”[55]
How to make testing easier
Advice from pediatric ophthalmologists that use RETeval in their daily practice

Involve parents, and let the child sit on their lap, they may hold it over the shoulder, whatever position is convenient for the child. Your RETeval can be used even upside down without compromising the quality of the test results.

As you test only one eye at a time, use the other eye to entertain the child: play a movie, use toys with bright colors and sound. Make a fun game out of ERG test time.

For small babies, use non-nutritive sucking and white noise – it really works!

We do our best to support doctors as we know that not everyone is familiar with executing ERG tests and interpreting results. That’s why we developed the Customer Resource Center which offers a wide range of materials created with the help of users from all over the world.
New to electrophysiology? Watch our “SunERGy” video series that gets you from zero to hero in ERG.
Need help understanding ERG results? Open the Clinical Interpretation Guide and the Case Book.
Do you want to tell parents about this new test provided by your clinic? Utilize the Practice Resource Kit we prepared for you.
All these materials, and many more, are available exclusively to LKC customers through the RETeval Academy.
Dr. Wendy Harrison, OD, Ph.D., FAAO, a professor at the University of Houston and an expert in visual electrophysiology, electroretinography, and pediatric eye care, describes what she loves about the non-invasive and child-friendly nature of the RETeval device.
Request a demo or a quote for the RETeval.
![]() Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Substance Tables:
The table below lists substances which may be contained within LKC’s RETeval and RETevet products. Substances listed as Type 1 are within permissible levels in one or more of LKC’s products. Substances listed as Type 2 are used in the production of some components used in LKC products and may be present at trace levels, but are typically destroyed during processing.
RETeval and RETevet Devices
| Substance | CAS # | Type | Listed as causing: |
| Nickel | 7440-02-0 | 1 | Cancer |
| Acrylonitrile | 107-13-1 | 2 | |
| Ethylbenzine | 100-41-4 | 2 | |
| Crystaline Silica | 14808-60-7 | 1 | |
| Lead | 7439-92-1 | 1 | Cancer Developmental Toxicity Male Reproductive Toxicity Female Reproductive Toxicity |
| Methylene Chloride | 75-09-2 | 2 | Cancer Female Reproductive Toxicity |
| Bisphenol A | 80-05-7 | 2 | |
| N-Hexane | 110-54-3 | 2 | Male Reproductive Toxicity |
