Enhance the Glaucoma picture Measure ganglion cell function
Early diagnosis is essential to preserve vision loss from further degeneration of ganglion cells.
However, Glaucoma is often challenging to diagnose and manage. Testing such as perimetry and OCT imaging are standard of care, but are all too often hampered by media opacities (imaging for cup-to-disc-ratio), unspecific testing methods (IOP), or subjective and bothersome to complete (Visual field test).
PhNR stands for Photopic Negative Response and is an electroretinographic test for analyzing how the ganglion cells function.
During the Photopic Negative Response test with full-field flash ERG, the retina is illuminated with a red flash on blue background under light-adapted conditions. This last negative peak is derived from the ganglion cells and their axons and is generally used to evaluate subjects for Glaucoma.
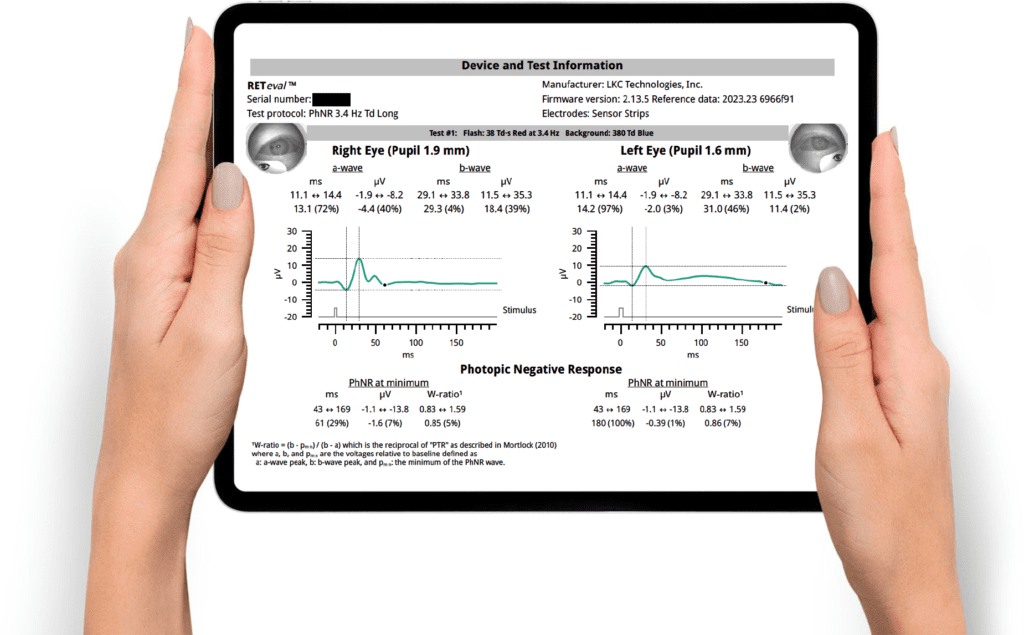
The PhNR response from ERG is described by a time (implicit time) and amplitude. For Glaucoma diagnosis and management, several parameters are given as the disease itself is complex [37].
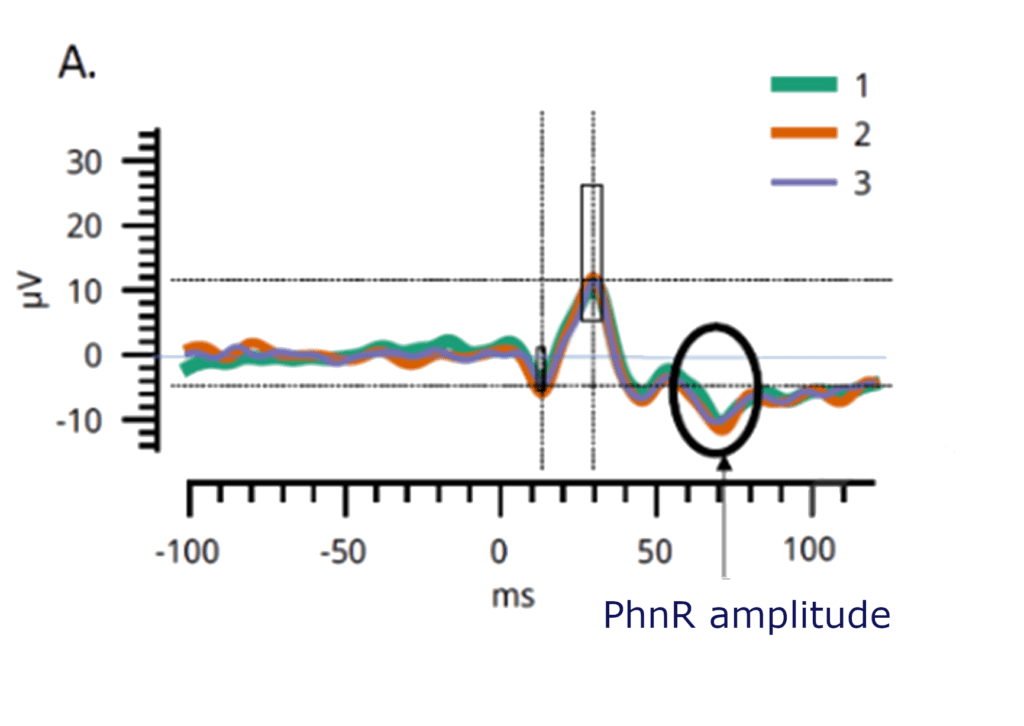
The RETeval PhNR test objectively measures the ganglion cells function by evaluating the electrical activity of the cells to a light stimulus. Knowing about the function of the ganglion cells assist in the detection and monitoring of glaucomateous changes in any patient.
The test provides high objectivity and repeatability, is independent from media opacities and is suitable also for patients with reduced motor and cognitive skills [31][32][34].

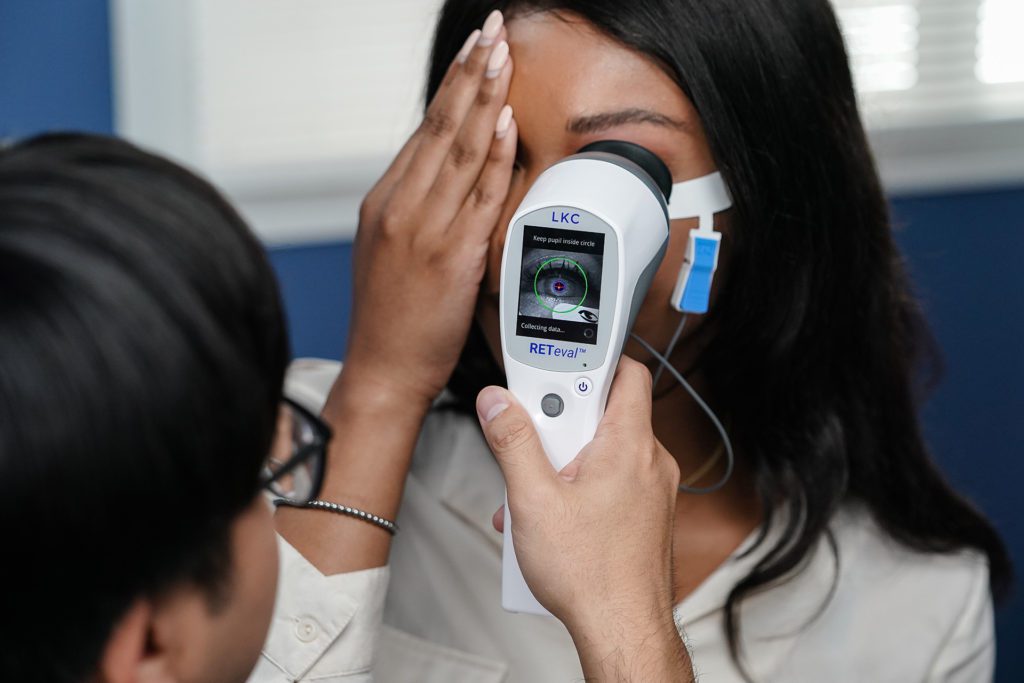
Real time pupillography adjusts for pupil size in real time - can be used in patients who are not dilated or in any stage of dilation

Our non-invasive LKC patented Sensor Strip skin electrodes mean no corneal electrode required

Handheld, light and portable.
Train technicians in minutes.
Mac and Windows compatible

The RETeval Device is expected to detect changes with a high degree of accuracy
(Early Glaucoma)
Analyze the PhNR Amplitude at minimum
(Moderate Glaucoma)
Analyze the PhNR W-ratio

PhNR testing with the RETeval Device provides sensitive, objective monitoring of retinal changes for more informed follow-up, even where no changes in visual field or RNFL could be detected
Hui et al. detected improvements in the inner retinal function after treatment with nicotinamide, where no improvements in visual field or RNFL had been seen [39].
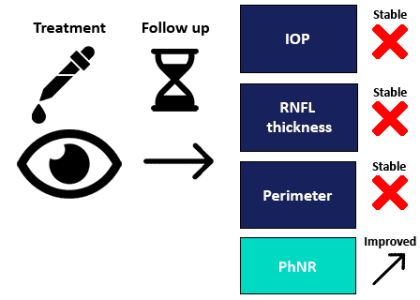
Our new algorithm provides a significant improvement in test/retest variability, leading to improved ability to track patient progress longitudinally. Additionally, the reference range is narrower, leading to an enhanced ability to aid in the detection of abnormal ganglion cell function.
The protocol is ISCEV* compliant, uses the most effective stimuli settings, and is available in different configurations that allow physicians to adjust according to patient conditions (short and long test options, mydriatic and non-mydriatic).
*International Society for Clinical electrophysiology of Vision




Request a demo or a quote for the RETeval
![]() Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Warning: This product can expose you to chemicals including lead, which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Substance Tables:
The table below lists substances which may be contained within LKC’s RETeval and RETevet products. Substances listed as Type 1 are within permissible levels in one or more of LKC’s products. Substances listed as Type 2 are used in the production of some components used in LKC products and may be present at trace levels, but are typically destroyed during processing.
RETeval and RETevet Devices
| Substance | CAS # | Type | Listed as causing: |
| Nickel | 7440-02-0 | 1 | Cancer |
| Acrylonitrile | 107-13-1 | 2 | |
| Ethylbenzine | 100-41-4 | 2 | |
| Crystaline Silica | 14808-60-7 | 1 | |
| Lead | 7439-92-1 | 1 | Cancer Developmental Toxicity Male Reproductive Toxicity Female Reproductive Toxicity |
| Methylene Chloride | 75-09-2 | 2 | Cancer Female Reproductive Toxicity |
| Bisphenol A | 80-05-7 | 2 | |
| N-Hexane | 110-54-3 | 2 | Male Reproductive Toxicity |
